Many studies on memory and learning utilize the immediate early gene c-Fos as an indicator of recent synaptic activity. By quantifying c-Fos positive neurons, we can compare activity across different populations in the brain, and analyze co-labelled neurons with other useful cell markers such as BrdU and Doublecortin.
Our lab recently devised an amplification protocol for c-Fos stains that specifically increases the intensity of the signal via a Biotin-Streptavidin-Rhodamine complex (see below). We have demonstrated this amplification protocol is effective over a wide range of primary antibody dilutions, with strong signaling from 1:250 to 1:5000 dilution. Basically, our amplification protocol is a fluorescent dream come true. But don’t just take our word for it, check it out for yourself. Click on images to biggify.
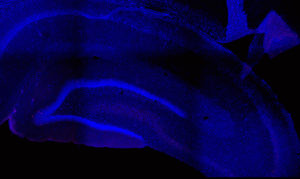
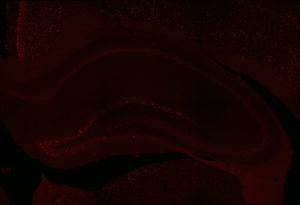
Fos 1:250 primary antibody dilution, without amplification:
Here you can clearly see some Fos+ cells, especially in the dentate gyrus, where signal is always greatest. But there aren’t many cells there, or in other regions. We don’t expect that many cells, because of sparse coding in the hippocampus, but still…
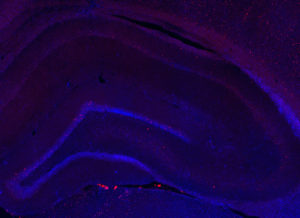
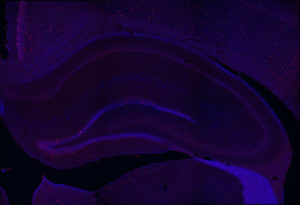
Fos 1:250 primary antibody dilution, with amplification:
Now we’re talking! Fos everywhere!
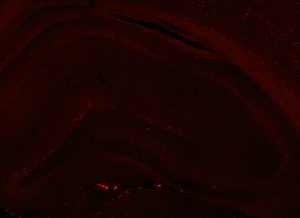
Fos 1:5000 primary antibody dilution, with amplification:
Basically the same as 1:250 except possibly less background staining, better signal to noise, and save $$$.
c-Fos Fluorescent Immunohistochemistry Protocol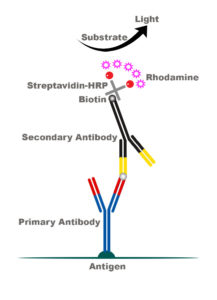
Day 1:
1) 3 x 5 min wash in PBS
2) 30 min wash 10% PBS-Triton X (TX) with 3% horse serum
3) Add primary antibody for 3-day incubation period (tested range of 1:250 to 1:5000 dilution of c-Fos antibody (Santa Cruz, sc-52-G) in 10% PBS-TX w/ 3% horse serum)
Day 2:
1) 3 x 5 min wash in PBS-TX
2) 1 hour at room temp Secondary Antibody (biotinylated anti-goat 1:250, Jackson Immuno #705-065-147, in 10% PBS-TX w/ 3% horse serum)
3) 3 x 5 min wash in PBS
4) 30 min Blocking Solution at 5%
a) To make blocking solution:
– 40 mL PBS
– 0.2g blocking powder (Perkin Elmer, FP1020)
– Warm to dissolve then cool to room temp before use
5) 1:100 Streptavidin Horseradish Peroxidase (Perkin Elmer, NEL750) in Blocking Solution for 1 hour
6) 3 x 5 min wash in PBS
7) 30 min 1:2000 NHS-Rhodamine (Fisher, PI-46406), in PBS w/ 1:20,000 H2O2
8) 2 x 5 min wash in PBS
9) 1 x 5 min incubation 1:1000 DAPI in PBS
10) 4 x 5 min wash in PBS
Staining Recommendation: Incubate any additional primary antibodies separately from amplification Day 2 after adding the Rhodamine (step 7) and completing 3 subsequent PBS washes.
Acknowledgements: Amplification protocol was devised by PhD student Shaina Cahill and undergrad student Angela Martinovic. Dilution fidgeting performed by MSc student Alyssa Ash. Wisdom, guidance and praise contributed by Jason Snyder.
This protocol is also published on Figshare, an open access medium to rapidly share scientific information:
Martinovic A, Ash A, Cahill SP, Snyder JS (2017) c-fos immunohistochemistry protocol. Figshare: https://doi.org/10.6084/m9.figshare.5229898.v1

Thanks for sharing! Could you please describe the concentration of NHS-Rhodamine?
I’d play around with it to see what works best in your hands…
Excellent work Jason. Has your group shifted to a new Fos (or Zif268) antibody since Santa Cruz no longer offers polyclonal antibodies. We have been unable to get their monoclonal Ab to work
We have not yet tried any other vendors’ antibodies but will soon and will share here! I did hear people on Twitter talking about Synaptic Systems Fos antibody working well…
Hi, Is that 10% TX-100 in PBS when you say “10% PBS-Triton X” in the protocol. I’m asking because that is incredibly high compared to standard IHC use of Triton X. It’s normally used between 0.1-0.4% so I just wanted to check that it really means 10% TX. I’d really appreciate any clarification. Thanks!
You’re right – it’s higher than usual. It really means 10%!
One more question: In step 7 where the NHS-Rhodamine in PBS is applied it is with 1:20,000 H2O2. Is that using the concentrated 30% H2O2 at that dilution or 7% H2O2?
Hi, I actually have a more general cfos question that perhaps you can answer since you seem to be such pros… I was wondering if you know whether cfos degrades over time after tissue has been fixed. We currently do behavior two animals at a time over the course of a couple of weeks, so all in all the entire experiment takes a few months. Would you recommend processing the animals as they are sacrificed, or until they have all been sacrificed in one large batch? Thank you in advance for your help.
I don’t think it will degrade significantly at all. I would process all the tissue at the same time since there can be differences in staining quality from one day to the next.
which animals have you successfully performed the cFos staining so far?
(just) mice and rats!