
It’s great to see this paper finally in print. At SFN 2008 the authors had a poster that generated a lot of excitement, at least in our circles. And the poster was quite a sight: there was such a profusion of data that the poster poured off the easel, nearly reaching the floor. With 27 (!) supplemental figures in the final article, one has to wonder if this is the final straw that led to this article.
The authors use an ingenious approach to address an idea that has been floating around for a while: that adult neurogenesis regulates memory turnover in the hippocampus. The hippocampus appears to have a temporary role in memory storage — for instance, lesions to the hippocampus often impair recall of recent memories, but have little or no effect on remote memories. One interpretation of this result is that memories are stored in the hippocampus for only a short time and then transferred to other brain structures (likely neocortex) in a process called systems consolidation. This may then free up space in the hippocampus for new memories. Neurogenesis could be a mechanism for this process: for instance, the addition of new neurons to the dentate gyrus of the hippocampus might destabilize old memories and simultaneously provide a fresh substrate for new memories.
-
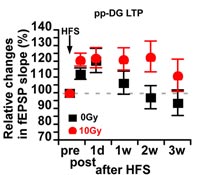
LTP lasts longer in irradiated rats (red circles) that lack adult neurogenesis.

LTP lasts longer in irradiated rats (red circles) that lack adult neurogenesis.
Kitamura et al performed a truly heroic number of experiments to provide evidence that adult neurogenesis is involved in memory turnover in the hippocampus. First, they examined whether neurogenesis is important for the persistence of hippocampal long-term potentiation (LTP) in rats. LTP is an activity-dependent increase in the strength of synaptic connections between neurons, and is studied as a cellular model of memory.
They showed that blocking adult neurogenesis via irradiation extends the duration of hippocampal LTP: LTP lasts less than 2 weeks in control rats but lasts more than 3 weeks in irradiated rats (left). Thus, at a cellular/synaptic level, it would seem that neurogenesis causes memories to be cleared from the hippocampus.
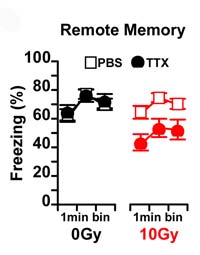
- Inactivating the hippocampus with TTX impairs memory in irradiated mice (red circles).

Then, in a series of fear conditioning experiments, the authors demonstrated that adult neurogenesis is in fact involved in the clearance of hippocampal memories in mice. In these experiments neurogenesis was blocked with irradiation or a genetic method prior to conditioning. On the surface, both control and neurogenesis-arrested mice acquired fear conditioning normally, and both groups remembered for at least a month. However, a critical difference emerged when the hippocampus was inactivated during remote recall testing: one month after training, hippocampus inactivation failed to impair recall in control mice, replicating a classic effect and suggesting the memory had been transferred to other brain regions. In contrast, in neurogenesis-arrested mice, hippocampal inactivation did impair recall of the remote memory, indicating that the memory still resided in the hippocampus. Importantly, hippocampal inactivation did impair recall in controls 1 day after conditioning, when memories had not yet been consolidated into the neocortex. Collectively, these findings suggest that adult neurogenesis contributes to the consolidation of memory to extra-hippocampal structures.
The authors then go ahead and answer some of the exciting new questions raised by these findings: For example, what happens if, instead of having reduced neurogenesis, you have increased levels of neurogenesis? Well, consistent with their hypothesis, mice that were given access to running wheels had increased neurogenesis (as expected) but as soon as 7 days after conditioning their memory was no longer hippocampal-dependent, unlike controls. Thus, increasing neurogenesis accelerated memory consolidation.
Another interesting finding was that the hippocampal-dependence of memory was prolonged when mice were irradiated only 11 days before conditioning. Since 11-day-old neurons do not even have functional (excitatory) synapses this suggests that it may not be functional young neurons present at the time of learning, but instead the synaptic integration of new neurons after learning that facilitates clearance of memory from the hippocampus.
Why else is this paper cool? Well, to date, adult neurogenesis has been implicated in many different types of behavior but it has been hard to identify common threads – this paper makes a new prediction that neurogenesis is specifically involved in consolidation. It also reconciles a discrepancy in the field – whether or not young neurons are involved in long-term memory. Some studies have found that reducing neurogenesis impairs long-term memory (Snyder 2005, Jessberger, 2009), but others have not observed this effect (Saxe, 2006). Kitamura’s data shows that even if long-term memory appears normal at a behavioral level, reducing adult neurogenesis will have altered the neural substrate of the memory. And so could these memories really be equivalent? This will be an exciting avenue for future studies to explore: how is hippocampus-dependent long-term memory (in neurogenesis-arrested animals) different from hippocampus-independent long-term memory?
It will also be interesting to further explore how arresting neurogenesis affects memory acquisition. If clearing memories from the hippocampus makes room for new memories, then shouldn’t the acquisition of new memories be hindered when clearance stops? Kitamura’s failure to observe significant impairments in memory acquisition may mean that neurogenesis is involved less in memory clearance than in reorganizing memory networks to include extra-hippocampal structures. That is, instead of simply expunging hippocampal memories, new neurons may facilitate the generation of additional memory representations in extra-hippocampal structures.
Another interesting issue is how the apparent give-and-take relationship between hippocampus and neocortex (presumably) arises. The data seem to suggest that the presence of a hippocampal memory representation prevents a cortical memory representation from arising. It’s as if the memory is a discrete entity that can be passed from hippocampus to cortex: as long as the memory remains in the hippocampus, the cortex is deprived. It would be great to know how –and why– this happens.
Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, & Inokuchi K (2009). Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell, 139 (4), 814-27 PMID: 19914173