Update: The poster is now available at Nature Precedings.
Still acquiring histological images for my SfN poster. My recurring problem is that I end up taking pictures of things because they’re pretty and not because they have anything to do with the task at hand. Today’s case in point:
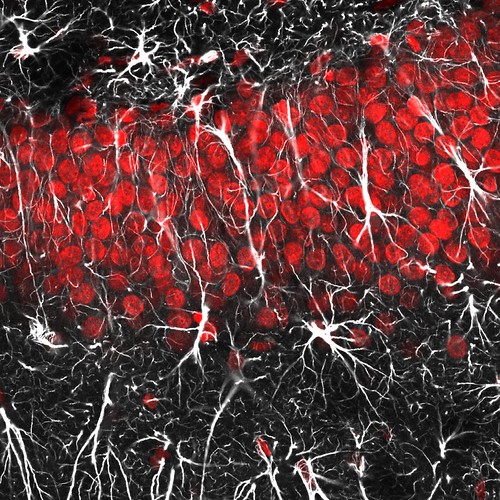 Well, this does relate to my SfN poster a little bit. Red shows cell nuclei, most of which are dentate gyrus granule neurons. And white is GFAP immunostaining, which largely labels astrocytes but in this part of the brain also labels radial glia, the stem cells (or to be less controversial, “precursor” cells) of the hippocampus. Radial glia can be identified by the long process (almost like a dendrite) that they extend through the granule cell layer. There are a few in the above picture.
Well, this does relate to my SfN poster a little bit. Red shows cell nuclei, most of which are dentate gyrus granule neurons. And white is GFAP immunostaining, which largely labels astrocytes but in this part of the brain also labels radial glia, the stem cells (or to be less controversial, “precursor” cells) of the hippocampus. Radial glia can be identified by the long process (almost like a dendrite) that they extend through the granule cell layer. There are a few in the above picture.
What I was really looking for was a photo more along the lines of this, which may appear on my poster if I cannot find a better (prettier) example in the next few days:
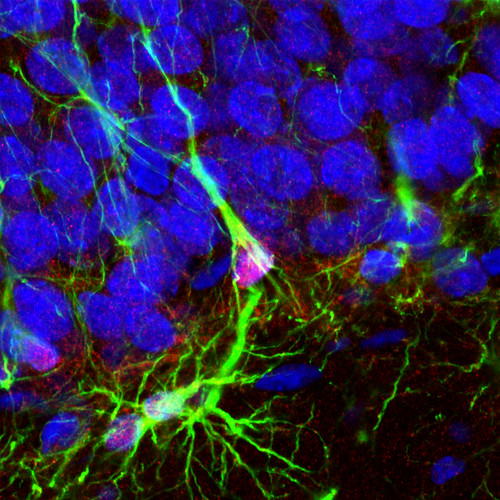 Here, dead center, is an example of a radial glial cell with a green GFAP process and a little bifurcating hat that sits on the cell body at the base of the granule cell layer. What’s special in this case is the fact that these are transgenic rats in which thymidine kinase is also expressed in the GFAP+ cells. By giving the rats “treats” laced with valganciclovir we can then specifically kill the radial cells, and prevent adult neurogenesis. This is the same strategy used to inhibit adult neurogenesis in several lines of mice. Now we can do it in rats too (which is great because rats have a bigger brain making them more amenable for some kinds of experiments, and they’re also smarter).
Here, dead center, is an example of a radial glial cell with a green GFAP process and a little bifurcating hat that sits on the cell body at the base of the granule cell layer. What’s special in this case is the fact that these are transgenic rats in which thymidine kinase is also expressed in the GFAP+ cells. By giving the rats “treats” laced with valganciclovir we can then specifically kill the radial cells, and prevent adult neurogenesis. This is the same strategy used to inhibit adult neurogenesis in several lines of mice. Now we can do it in rats too (which is great because rats have a bigger brain making them more amenable for some kinds of experiments, and they’re also smarter).
To hear the full story stop by the poster on Saturday. Actually, stop by regardless of whether you want to see the poster. Isn’t that how SfN works?
Here is the abstract:
A transgenic rat model for reducing adult neurogenesis. Saturday, Nov 12, 2011, 1:00 PM – 2:00 PM. 30.05/A27
JS Snyder, L Grigereit, M Brewer, J Pickel, HA Cameron. NIH/NIMH, Bethesda, MD, USA
Our understanding of the function of adult neurogenesis is largely founded on animal models. Models of reduced neurogenesis, in particular, suggest new neurons contribute to various aspects of learning, memory and emotional behavior. While many different methods for reducing adult neurogenesis exist in the mouse, fewer tools are available in the rat. Additional means for reducing neurogenesis in the rat would be useful if they offer additional specificity over existing methods (e.g. irradiation or chemical) and do not require special equipment (e.g. irradiator). Since the rat offers certain advantages for behavioral neuroscience studies (complexity of behavior, brain size, extensive literature) we sought to develop a genetic method for reducing neurogenesis in this species. Specifically, we created transgenic rats expressing herpes simplex virus thymidine kinase (TK), which renders mitotic cells sensitive to the antiviral drug ganciclovir. TK was expressed under control of the GFAP promoter, thereby allowing for the selective killing of GFAP+ radial precursors in the adult brain. Wild-type and GFAP-TK rats were treated with 7.5mg of orally-available valganciclovir daily for 5 days each week and injected with the cell division marker BrdU after 1, 2, 3, or 4 weeks of treatment. GFAP-TK rats gained weight normally and appeared healthy throughout treatment. Four weeks after the last group received BrdU all rats were perfused. Consistent with a role for GFAP+ radial precursors in generating new dentate granule neurons in the rat, BrdU+ granule cells were reduced by 78% after 1 week of valganciclovir treatment and ~85% thereafter in GFAP-TK rats compared to wild types. Current experiments are exploring neurogenesis reduction in the subventricular zone-olfactory bulb of GFAP-TK rats, another brain region known to give rise to adult-born neurons via GFAP+ precursors. Given the extent of adult neurogenesis reduction in the dentate gyrus, we expect that these rats will be a valuable tool for investigating functions of neurons born in the adult brain.
Very pretty pictures – I like the white stain particularly 🙂
So I’m interested in these radial glia as neuronal precursors – I didn’t know that dentate gyrus neurons were glia beforehand. I’d love to learn more! Is there a percentage of these glia that don’t differentiate into neurons? Does this differ in the SVZ vs the dentate gyrus? And is there some functional role that these glia carry out before they differentiate into neurons, like guiding migration (as you see in the cortex)?
Jason, as usual spectacular images. We are producing enriched vGluT2+ neurons from suspension cultured ES cells for neurotox type of studies. Immediately after differentiation and plating we see a radial glial-like morphology, followed by development of traditional pyramidal neuron-like characteristics. In the first few days we see what *appears* to be co-expression of GFAP and b3 tubulin in many of these and are now trying to identify if the GFAP signal is real, or an artifact. Have you co-stained radial glia in situ for putative astrocyte and early neuronal markers? If so, what have you observed?
I haven’t done that myself but I did see recent paper by Seki that describes something similar, using GFAP and the neuronal marker Hu: http://www.ncbi.nlm.nih.gov/pubmed/21966492 and http://www.ncbi.nlm.nih.gov/pubmed/20026190
That first paper is great. We’re seeing something similar, in that we have a mixed population, so that is encouraging. Not sure how Tubb3 and Hu expression related, but that’s something we can figure out…
thanks!
patrick
That top one is very pretty.
My daughter will be graduating with a neuroscience degree and is hoping for a career in neuroradiology. I am wondering if you would consider selling any of your images as prints for framing??? (looking for Christmas or graduation gifts) Let me know. Thanks.
Pingback: Antigen and Antibody: Celebrity Couples in Science | everydaybiochemistry