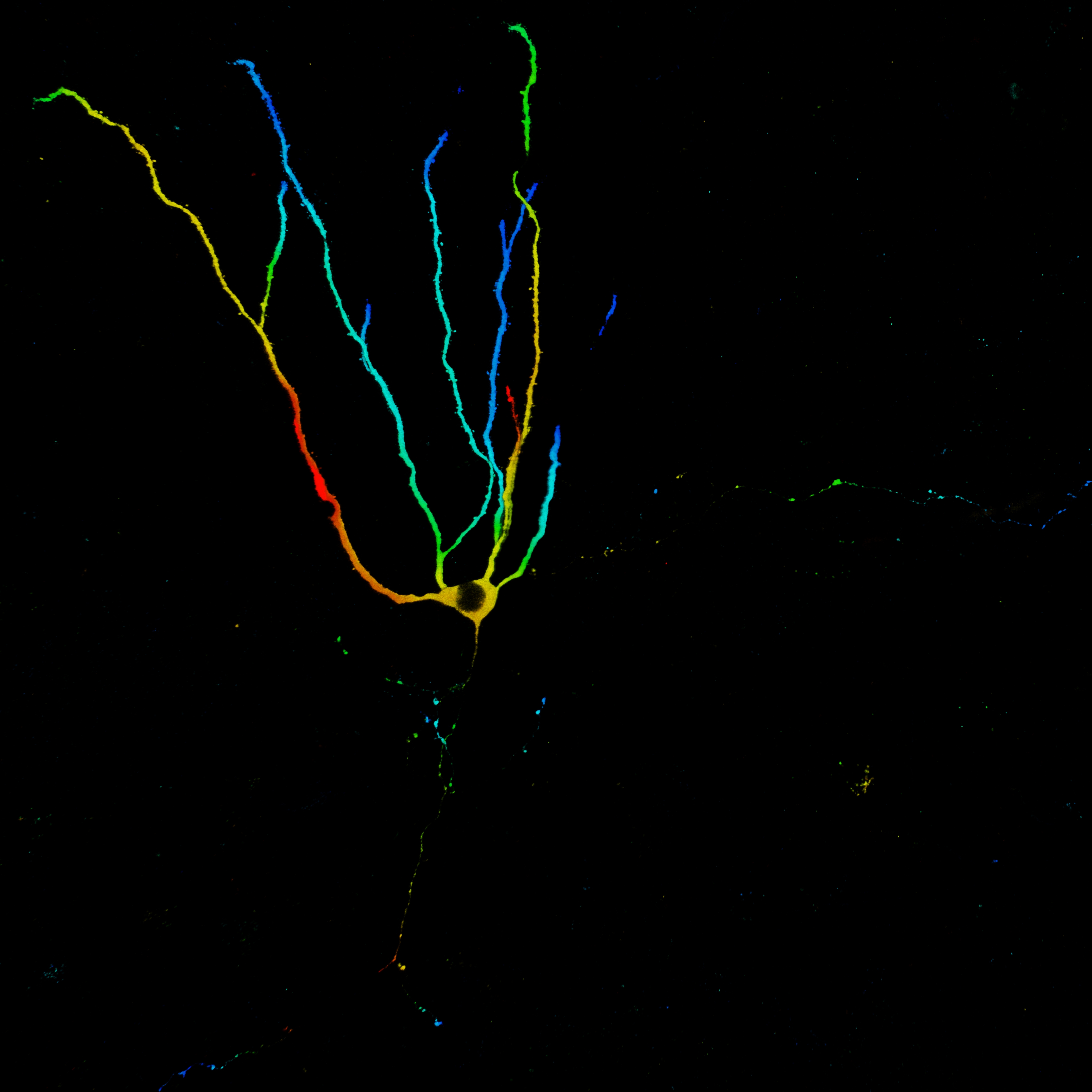
 Retroviruses can infect actively dividing neural progenitor cells in the hippocampus and spare non-dividing cells such as mature neurons. With this tool we can selectively label newly born neurons. We can infect neuronal precursor cells at various stages in development and look at a neuron’s morphology at certain ages. This will give us insight about integration of newborn cells in the neuronal network and we can examine how external influences such as stress or learning affect the morphology of a newly born neuron.
Retroviruses can infect actively dividing neural progenitor cells in the hippocampus and spare non-dividing cells such as mature neurons. With this tool we can selectively label newly born neurons. We can infect neuronal precursor cells at various stages in development and look at a neuron’s morphology at certain ages. This will give us insight about integration of newborn cells in the neuronal network and we can examine how external influences such as stress or learning affect the morphology of a newly born neuron.
We use the retroviruses not only to label newborn neurons but also to manipulate them by using viruses encoding for optogenetic proteins or DREADDs. These methods allow us to activate or inhibit targeted neurons either acutely or chronically and investigate the resulting consequences on neuronal morphology and behaviour.
We would like to thank Diane Lagace, Shaoyu Ge, Bryan Luikart and Ana Martin-Villalba for providing cells and viral vectors for virus production in the Snyder lab!
This movie (note available in HD!) shows 2 neurons in the rat dentate gyrus labelled on postnatal day 1. The green/GFP-labelled neuron also expresses the inhibitory DREADD receptor HM4Di and the red cell expresses RFP. Cells were imaged and movified on our Leica SP8 confocal microscope. Note the long and divergent axon collaterals of the red cell. One travels extensively throughout the granule cell layer. What is it doing there? Synapsing onto adult-born neurons?
This second movie shows a short dendritic segment and associated spines, putative sites of synaptic contact from incoming axons, obtained at high magnification. Since neurons are cut as the tissue is sectioned we’re aiming to image thicker sections so we can get the whole neuron in the same piece of tissue, but still have sufficient quality for imaging fine detailed such as spines and presynaptic terminals. Our first attempt was to cut sections at 100µm (uncleared tissue) and both the overall morphology and fine detail look pretty good!