Two recent papers have attracted a lot of media attention because they draw direct links between adult neurogenesis and behavioral disorders: Noonan et al. showed that rats lacking adult neurogenesis (stopped with irradiation) are more susceptible to cocaine addiction. Jin et al. showed that mice lacking adult neurogenesis (using a transgenic model) suffer greater infarct size and have more severe motor deficits after stroke.
While the papers themselves have important implications, what caught my attention was the angle taken by press releases: both articles studied the effects of reducing neurogenesis but the media focused on potential benefits of increasing neurogenesis. See speculation that antidepressants, by increasing neurogenesis, might be stroke-protective here. And, from Science Daily:
While the research specifically focused on what happens when neurogenesis is blocked, the scientists said the results suggest that increasing adult neurogenesis might be a potential way to combat drug addiction and relapse.
It may very well be the case that increasing neurogenesis is good in the same way decreasing neurogenesis is bad but it shouldn’t be assumed – maybe we have all the neurogenesis we need and, while completely arresting neurogenesis could be harmful, increasing neurogenesis beyond normal levels is just redundant.
Or, maybe the key is where you’re increasing or decreasing neurogenesis from:
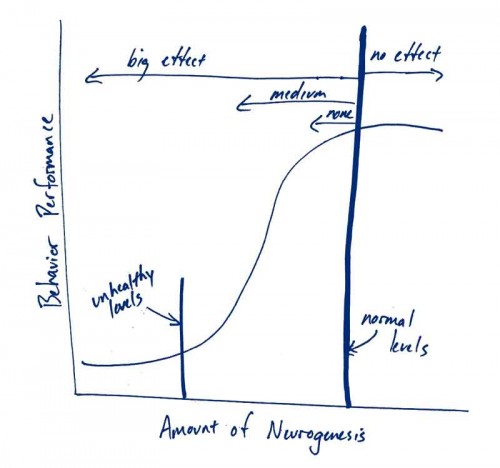
Figure 1: Decreasing neurogenesis in the healthy brain, but increasing neurogenesis in the unhealthy brain, may alter behavior
In this model, assuming most of us have normal levels of neurogenesis, further increases will provide no benefit to behavioral performance, whether we’re talking resistance to addiction or recovery from stroke or anything else. However, decreases in the number of young neurons below a critical level would impair storing/processing of information, protection against stroke etc.
This speculation is under the assumption that we all have “normal” or sufficiently high levels of constitutive neurogenesis. However, in the case of addiction, covariables like stress, poor nutrition and narcotics themselves might all serve to reduce neurogenesis below healthy levels (see “unhealthy levels” in figure). In this case, increasing neurogenesis would cause dramatic improvements in behavior and is consistent with the authors’ speculation. For similar reasons, increasing neurogenesis might provide cognitive benefits to people in other situations where neurogenesis is known to be compromised: the aged, those experiencing chronic stress, patients undergoing cranial irradiation or chemotherapy.
It may be surprising that this idea is actually very hard to test, even in the laboratory. While many tools exist for reducing neurogenesis to unhealthy levels (irradiation, antimitotic drugs, genetics) there are no tools for selectively increasing neurogenesis beyond normal/healthy levels. Yet.
References
Noonan MA, Bulin SE, Fuller DC, & Eisch AJ (2010). Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience, 30 (1), 304-15 PMID: 20053911
Jin K, Wang X, Xie L, Mao XO, & Greenberg DA (2010). Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proceedings of the National Academy of Sciences of the United States of America PMID: 20385829
Dear Jason.
I have been reading extensively this entry of yours and although I agree in the point you are trying to make (i.e. decreased neurogenesis as opposite to increased neurogenesis, both compared to basal levels) I am not sure I absolutely agree with your affirmations. First of all, there ARE means to increase neurogenesis beyond normal levels: running; and even beyond “healthy” levels (whatever that means). For instance, almost everything that induces seizures affecting the hippocampus (i.e. kainic acid) will increase neurogenesis as compared to basal levels (i.e. Jessberger S, et al., J, Neurosc., 2007) and particularly in this model it has been demonstrated that too much neurogenesis is actually deleterious to the hippocampus.
All in all, I think that it would be quite safe to speculate that, to the contrary of what the press suggested, the problem is not as simple as it looks like ( is ever?) and that there is a adequate physiological level of neurogenesis that whether decreased or increased will affect its normal functions.
So, one should aim to revert or correct neurogenesis (no normal, relative levels) but never to increase or decrease it (in absolute terms) . Based on this I would suggest that your model needs to adjusted to a inverted U-shaped curve.
I will be very happy to hear your opinion on this
Carlos
Hey Carlos – I wasn’t super clear there at the end – I do agree there are ways to increase neurogenesis but the key is *selectivity*. Running increases neurogenesis but it has so many other effects on the brain that you can’t isolate the neurogenesis-specific benefits.
Your ideas with respect to seizures bring up a good point – that greatly-increased neurogenesis could be bad. Or maybe too much neurogenesis in an unhealthy (e.g. epileptic) brain is bad (creating a U-shaped curve) but in a healthy brain has little effect (creating the sigmoidal curve shown so artistically, above).
(BTW, I would argue the Jessberger paper doesn’t show increased neurogenesis is detrimental – they just show that new neurons are integrating differently in the epileptic brain. This altered integration could certainly be bad but, as Jakubs 2006, speculated, it may actually be an attempt to homeostatically restore normal functioning. I feel like someone *must* have looked at seizure susceptibility in normal and neurogenesis-deficient animals…)
Thanks for the ideas!
Jason
Hey Jason,
to continue with the discussion “more-neurogenesis-is-not-always-good”, it would be interesting to note that in animal models of Alzheimer’s , increased proliferation and in some cases increased survival of new neurons has been observed (Haughey 2002 (2x) and Mirochnic 2009, a.o.). I shall say these observations add to the idea of the need to restore neurogenesis levels to normal (whatever that may be) instead of increase or decrease it in absolute terms. What rises the question of how to define normal levels, of course. This is, how (or where) would you place the y=0 in your graph above?
Cheers,
Carlos
Definitely there’s no absolute number of new neurons that is ideal for each animal but instead I’d propose that the optimal number of new neurons depends on how many the animal needs to maintain some sort of behavioral homeostasis. For example, say that learning increases neurogenesis and that these new neurons take part in storing this new information permanently. You could argue that as an animal ages it learns more and more about the things of the world, and less and less exists for that animal to learn about in the future. And so neurogenesis is reduced accordingly. An animal that learns more than average during early life may therefore have reduced neurogenesis later in life (and vice versa) – their optimal levels of neurogenesis are different. Take this sort of thinking and apply it to stress, nutrition, social factors etc. and you’ve got a lot of hard-to-tease-apart variables controlling the levels of neurogenesis!
Hi Jason, I have an question for you. So basically your theory states that if brain exposed to high levels of stress would be also exposed to excessive neurogenesis enhancement
(by medications, cannabis etc;) in right areas of the brain it may cause neuronal growth which would positively affect behavior, memory and learning. Is there anything you want to add about this particular paragraph?
Thanks. Mr.X
Mmmm, no.