The idea that adult neurogenesis protects individuals from depression is perhaps the single greatest motivator driving neurogenesis research. Not surprisingly, “neurogenesis depression” is the most common behavioral keyword that brings people to this blog (followed closely by “pattern separation”). So I’m excited to say that we will soon be publishing what (I think) is the best evidence that impaired adult neurogenesis actually causes depressive symptoms (in mice). The neurogenesis-depression hypothesis is over 10 years old and yet there is largely only correlational evidence linking neurogenesis to depression and no direct evidence that impaired adult neurogenesis leads to depressive symptoms. Naturally, this has led to skepticism (e.g. see this paper by Robert Sapolsky, and discussion by fellow bloggers: scicurious, neurocritic, neuroskeptic). A key factor in our study was stress: mice that lacked neurogenesis often seemed very normal when they were happily going about their business (as in previous studies by other groups). However, following stress, mice lacking neurogenesis had elevated levels of stress hormones and they also showed more depressive behaviors (or depressive-like, if you prefer). I hope to go into more detail soon.
For now, here is the abstract:
Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Jason S. Snyder, Amélie Soumier, Michelle Brewer, James Pickel & Heather A. Cameron. National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland, USA.
Glucocorticoids are released in response to stressful experiences and serve many beneficial homeostatic functions. However, dysregulation of glucocorticoids is associated with cognitive impairments and depressive illness. In the hippocampus, a brain region densely populated with receptors for stress hormones, stress and glucocorticoids strongly inhibit adult neurogenesis. Decreased neurogenesis has been implicated in the pathogenesis of anxiety and depression, but direct evidence for this role is lacking. Here we show that adult-born hippocampal neurons are required for normal expression of the endocrine and behavioural components of the stress response. Using either transgenic or radiation methods to specifically inhibit adult neurogenesis, we find that glucocorticoid levels are slower to recover after moderate stress and are less suppressed by dexamethasone in neurogenesis-deficient mice than intact mice, consistent with a role for the hippocampus in regulation of the hypothalamic–pituitary–adrenal (HPA) axis. Relative to controls, neurogenesis-deficient mice showed increased food avoidance in a novel environment after acute stress, increased behavioural despair in the forced swim test, and decreased sucrose preference, a measure of anhedonia. These findings identify a small subset of neurons within the dentate gyrus that are critical for hippocampal negative control of the HPA axis and support a direct role for adult neurogenesis in depressive illness.
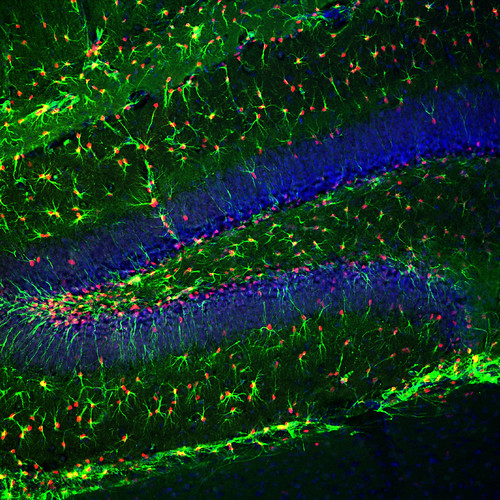
*image is of GFAP-driven thymidine kinase in a mouse brain (GFAP in green and thymidine kinase in red). In the presence of ganciclovir, any cell that expresses thymidine kinase dies when it attempts to divide. In this case those cells would be the radial glial stem cells that produce new neurons. These were the mice used to stop neurogenesis in the majority of the experiments.
UPDATE: Ed Yong at Discover Magazine and Scicurious at Scientific American have great summaries of the findings and their significance. And the Drugmonkey blog attacks the question of whether or not a depression study in mice can be relevant for humans.
Congratulations Jason! Let us know when it’s in press.
Dear Jason,
Congrats for the paper ! – as well as for all your blog, it is awesome.
I am raring to read your paper to know more about the experiments done. It will probably provide a step further in understanding the relationship between neurogenesis and stress-related disorders.
Reading the abstract, I have felt your paper closely echoes our recent “Molecular Psychiatry” article – http://www.nature.com/mp/journal/vaop/ncurrent/full/mp201148a.html. As a main difference, we never found an effect of ablated neurogenesis in control animals, even after an acute stress (see for example Suppl Fig S3C in the Santarelli’s paper – http://www.sciencemag.org/content/301/5634/805.abstract). In our experiments, mice always needed to be exposed to chronic stress to highlight the involvement of neurogenesis in the regulation of the stress response and only following antidepressant treatment. Particularly, we directly injected dexamethasone in hippocampus and tested how hippocampus itself modulates HPA axis and several downstream brain structures involved in the stress response.
Moreover, some results described in your abstract seem to be reminiscent of the previously published Schloesser’s paper (CORT increase after acute stress in mice with inhibited neurogenesis – http://www.ncbi.nlm.nih.gov/pubmed/19322118)
I have been quite surprised that you did not mention these two papers in your post. With the sole abstract it is difficult to know, but what do you think in your results is the most newsworthy, particularly in relation to the previous mentioned papers?
By the way, I am keen to look at the full paper and see the detailed results. In any case, your article provides new supports to the idea that adult neurogenesis is important for multimodal integration, specifically for stress integration and coping strategies during stressful experiences. This is probably the key to understand how disruption in neurogenesis may contribute to depression-like state and prevent recovery following antidepressant treatments.
Cheers
Alex – thanks very much for the comment and kind words. I am well aware of your recent paper and think it provides great support for the neurogenesis hypothesis of depression, inasmuch as it further strengthens the link between neurogenesis and antidepressants. I think that is the key difference with our study, that we found a role for new neurons in depressive behavior even in the absence of drugs. And I think this makes sense – if new neurons play a role in modifying emotional circuits following antidepressant treatment, shouldn’t they also play a role in the undrugged state?
While some people have looked for acute stress effects in neurogenesis-deficient mice (eg Santarelli as you mentioned) it seems to me it was never explored very thoroughly, and so maybe that’s why it was not previously identified. In our hands, following mild stressors such as bright open field exploration, neurogenesis-deficient mice had normal HPA regulation. After restraint, we observed a deficit, but it was transient (and could have easily been missed if we didn’t look at the right time points). Indeed, Schloesser found an HPA effect but in their experiments neurogenesis-deficient mice had higher cort even at baseline, something we never observed.
If you’ve read my previous posts, you’ll notice that I often tend to write an exhaustive summary and cite all of the relevant literature. I don’t have time to do this as much anymore! So I thought I’d just make this post more of a press release and instead just cite nothing. Thanks again for your comment and look forward to talking more – the paper comes out this week.
So does this lead to a new theory of the etiology of depression or just provide a physical explanation of the old “neurotransmitter imbalance” theory? In either case, does it imply new possibilities for treatment?
I’ve been through most of the meds (and various combinations of same) designed to change the amounts of various chemicals in my brain, but none have ever worked for very long. Working my way through the MOAIs now. Should these also not work, I would love to have something else to try before giving ECT a go.
The theory is not so new, that new neurons could help fight depression. It’s just that there’s not been much strong evidence in support of it. The big big challenge, in terms of making this significant, is understanding how this translates from animals to humans. We know very little about neurogenesis in humans and it will be very hard to find out if neurogenesis has the same function in humans as in animals (in this case, mice). There’s certainly a good chance it does and, so, the thinking has been that things like antidepressant treatments and exercise might also increase neurogenesis in humans and provide similar benefits as in rodents. There are companies that are developing drugs that specifically increase neurogenesis, in the hopes that they’ll be effective antidepressants. Offhand I’m not sure whether they’ve made it to clinical trials or it’s still in earlier stages…
Does “neurogenesis” include creation of glia or is that a completely separate mechanism?
“Neurogenesis” refers to creation of neurons; “gliogenesis” refers to the creation of glia…
While I am excited about the study done with mice, and I know that folks need help with depression, I also believe that humans have thoughts that compete with the appetite of depression. Mice, not so sure.
I work with people daily, and I say, when you meditate you focus on positive thoughts (perhaps), develop the parasympathetic muscle by slowing down (perhaps), but I realize that 20 minutes of meditation compared to day’s worth of thoughts and environmental input – is a lot of data. Furthermore, if depression and its accompanying thoughts have been running the gamete of the roller coast of the CNS, they dominate – until stark intervention resuscitates the body. Yes, exercise helps, it does, but what happens when its over.
We need a treadmill for “thoughts” and we need to sweat them out.
So if you take out the neurons with corticosteroid-receptors, coticosteroid levels remain higher for a longer period of time? wow, just wow
But it’s probably still a chronic stress response that degenerates hippocampal neurons (in any wt strain) in the first place, isn’t it?
In response to your first point, we’re just removing *one* of the populations of neurons – the other mature granule neurons are still present and have corticosteroid receptors. It’s interesting though, that a relatively small population of granule neurons (the young ones) can have a significant effect.
For your second point – that’s an interesting speculation, that chronic stress could reduce neurogenesis, which, in turn, could lead to increased HPA activity, thereby further decreasing neurogenesis etc etc and ultimately leading to depression. Alternatively, other factors could decrease neurogenesis to initiate the cycle, and not just chronic stress…
The comment about chronic stress reducing neurogenesis isn’t a speculation, isn’t it? Actually, from my experience, most of studies investigating reduced neurogenesis and disregulation of HPA axis uses daily maternal separation as a chronic stress model… or do you view it differently?
Pingback: Dr. Isis Issues Some Ad Hominems For Those Who Hang Their Hats on Rats (or Mice) [On Becoming a Domestic and Laboratory Goddess] - Your Web Spin
Very interesting.
From the abstract you say that in neurogenesis-suppressed mice, dexamethasone suppression of CORT is reduced. However this can’t be a direct effect of anything going on in the hippocampus because CORT doesn’t come from the hippocampus it comes from the hypothalamo-pituitary axis.
Now an interesting thing about the HPA is that it’s under strong control of endocannabinoids. e.g. there’s a very nice paper showing that endocannabinoid signalling modulates ACTH release at the level of the pituitary and others recently showing that it’s actually endocannabinoids that drive the well known circadian variation in CORT.
http://www.jneurosci.org/content/23/12/4850.short
http://endo.endojournals.org/content/151/10/4811.short
So I wonder whether neurogenesis affects the HPA via endocannabinoids?
It might not, but endocannabinoids do seem to be emerging as extremely important in this system so it would be nice to see, for example, whether the negative effects of lacking neurogenesis are reversible by cannabinoid drugs.
Or whether they are exacerbated by CB1 antagonists.
Actually that would be a nice experiment. Just give neurogenesis-lacking animals CB1 antagonists. In wild type mice this causes anxiety and appetite loss.
http://journals.lww.com/behaviouralpharm/Abstract/2003/12000/The_cannabinoid_CB1_antagonists_SR_141716A_and_AM.2.aspx and many others
Give it to neurogenesis-lacking mice and t might make them even worse. But it might not do anything implying that they already lack cannabinoid signalling. And that would be very interesting.
Hey – thanks. Regarding the DEX experiment, the thinking was that if a rise in glucocorticoids is detected by the hippocampus (new neurons is particular), then giving artificial glucocorticoids should inhibit the endogenous release of corticosterone, by inducing negative feedback (since the hippocampus is a part of the greater hippocampal-hypothalamo-pituitary axis). The real reason why it couldn’t have been due to anything in the hippocampus, however, is because DEX doesn’t cross the blood brain barrier. Instead, we think that a chronic disturbance of the hippocampal regulation of the HPA axis could lead to dysregulation further downstream (eg at the level of the pituitary).
Will think on the endocannabinoid-glucocorticoid interaction, completely unexplored in terms of neurogenesis…
Since 1965, the dominant theory for both the causation and treatment of depression has been the “monoamine hypothesis” and many practicing clinicians are uninformed about the “neurogenesis hypothesis”. Since transcranial magnetic stimulation (TMS) has become the primary focus of my practice, I have followed the research in this area and am pleased to see that there is now direct evidence to support the “neurogenesis hypothesis”. As demonstrated by Ueyama and others (http://www.ncbi.nlm.nih.gov/pubmed/21265939), like antidepressant medication, TMS appears to produce its antidepressant effects by stimulating hippocampal neurogenesis indirectly via stimulation of the prefrontal cortex. Your paper is an important contribution to a growing body of evidence that is leading to a fundamental shift in thinking about the causation and treatment of depression.
Pingback: Brains that can’t grow new neurons are vulnerable to depression | Nutrition News
Hi Jason,
Great blog, and congratulations on the paper! I used to work with models of depression/ADTs and look at adult neurogenesis. This paper is great, as a big open question is answered, and a much needed link is established.
What are you thoughts in the other direction, with enriched environment or exercise in terms helping this neurogenic buffer and helping combat stressful stimuli?
Best wishes,
Krishna
Hi Krishna – increasing neurogenesis could help, though I’ve speculated before on the possibility that the effects of increasing neurogenesis may not be as simple as being the opposite of the effects of decreasing it. On the other hand, I don’t think environmental enrichment or exercise can really be a bad idea!
HI Jason,
I thought much the same. Even though there is quite some amount of supportive data suggesting more=better and less=bad. I agree it may not be all that simple. More could mean encryption of a ‘bad context’ and less might just be adaptation of some sort in some cases.
Congrats on the paper Jason! Don’t forget the little guys when you become a faculty member.
Enjoyed the paper…and your review was fun too (nice linking to the blog).
If you haven’t seen it yet, I found this article interesting:
http://www.ncbi.nlm.nih.gov/pubmed/21878528
Basically its saying that running is a critical part of environmental environment’s effects on neurogenesis. I’d be interested to know if running/swimming also underlies the effects of other treatments (such as water maze training, etc.) too. I’m wondering if there is more of a common mechanism and if these stimuli are more similar than we realize.
Has Reif et al
http://nervenklinik.uk-wuerzburg.de/fileadmin/uk/psychiatrie/Dokumente/Forschung/Psychiatric_Neurobiology_and_Bipolar_Disorder_Program/Adult_neurogenesis_in_schizophrenia
Reif et al 2006
been forgotten , demolished, rejected, not replicated, found wanting, or just ignored
I think one issue that clouds the interpretation of that study is the fact that a number of subjects were excluded for having too much neurogenesis…
Five cases were removed – made up from each of the sample i.e at most three from schizophrenia group of fifteen
I can’t understand – I’m a family carer – wanting some hope why there has been no demadn for repeating – confirming or denying.
I find subsequent studies like these give corroboration to a working memory reduction which REif et al would predict
Gold:- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2883794/pdf/nimhsi71564.pdf
Raalten:- http://www.ncbi.nih.gov/pubmed/18155446
היי אני חושב להראות לכל הבלוגרים, על אתר בתחום יצור ארונות אמבט וארונות בגדים וארונות ישירות מהמפעל ללא כל פערי תיווך .
One of the previous posters said that meditation was about focusing on positive thoughts. This is,simply not true. Meditation is just intentional non-judgemental awareness of the present moment. The whole point is that you don’t judge thoughts or sensations as good or bad.
This training can lead to a permanent state of meditation, which will break the biggest cause of depression, i.e. rumination.
Jason,
I just recently (8/13) came across your paper. I’m very interested in finding out as much as I can about Major Depression and the possible symptoms, causes, effects and possible solutions. I find the whole neurogenesis hypothesis extremely intriguing. Any new insights/info. since you released your paper?
Thx in advance,
Peggy N.