I’ve previously written about the functional differences between the septal (aka dorsal aka rostral¹ aka posterior²) and temporal (ventral/caudal/anterior) hippocampus and how studies are increasingly not treating the hippocampus as a single homogeneous structure. Myself and others have extended this perspective to studies of adult neurogenesis and now I’m happy to report that we had a new paper come out on the topic last week.
The study was a bit of a fun learning experience for me for several reasons. As many of you know I recently changed labs and will be starting my own lab soon. So things are on the go and I haven’t had the time to dive deep into a study that is going to take several years to complete. But some research projects can be done quickly and still are able to produce very useful results. As I prepare for my own lab I was probably thinking, “What kind of projects could a Master’s student accomplish??”. And indeed we had a strong postbaccalaureate fellow in the lab for about a year who fit this description pretty well (she’s the middle author). Also, we had lots of tissue remaining from a recent study where we compared neurogenesis in mice and rats that could be used to answer other questions, thereby saving time, money and importantly, animals. So we decided to ask whether the maturation and survival of adult-born neurons differ between the aforementioned functionally-distinct hippocampal subregions.
The basic idea of the study.
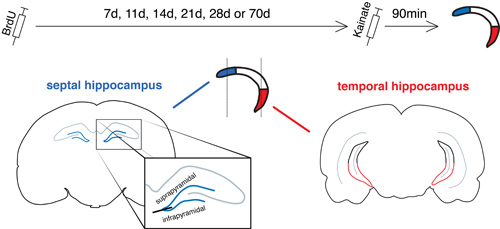 BrdU was injected to birthdate+label new neurons and at various intervals kainate was given to “activate” all the neurons that had integrated into the circuitry, and formed synapses. This integration (a major step in the new neuron maturation process) can then be measured by immunohistochemically staining for gene products such as Arc that are expressed after synaptic activity. Our tissue was cut in the coronal plane, however, which is not ideal for isolating the septal and temporal ends of the hippocampus (though others have found that there are meaningful differences along this rostrocaudal axis). Since the rostral sections are purely septal (see blue portions, above) this subregion was not a problem. We then decided to basically “cut” the caudal sections in half and by analyzing only the ventral portion we were able to specifically target neurons located in the far temporal dentate gyrus (shown in red). We were also able to investigate whether new neurons are more likely to survive in one subregion than another, by counting the number of cells present before (at 7d old) and after (at 28d old) the period of cell death.
BrdU was injected to birthdate+label new neurons and at various intervals kainate was given to “activate” all the neurons that had integrated into the circuitry, and formed synapses. This integration (a major step in the new neuron maturation process) can then be measured by immunohistochemically staining for gene products such as Arc that are expressed after synaptic activity. Our tissue was cut in the coronal plane, however, which is not ideal for isolating the septal and temporal ends of the hippocampus (though others have found that there are meaningful differences along this rostrocaudal axis). Since the rostral sections are purely septal (see blue portions, above) this subregion was not a problem. We then decided to basically “cut” the caudal sections in half and by analyzing only the ventral portion we were able to specifically target neurons located in the far temporal dentate gyrus (shown in red). We were also able to investigate whether new neurons are more likely to survive in one subregion than another, by counting the number of cells present before (at 7d old) and after (at 28d old) the period of cell death.
Since this article is completely open access you can see the actual data for yourself here. The short story is that new neurons matured faster in the septal dentate gyrus (all cells could express Arc by 3w of age whereas in the temporal dentate gyrus this didn’t occur until somewhere between 4-10w of age). The other new finding was that there were many more new neurons initially added to the infrapyramidal blade of the dentate gyrus but these neurons were much less likely to survive than neurons born in the suprapyramidal blade.
What is the significance of these findings? To me, they provide a framework for future studies. If you want to investigate new neurons specifically during their immature stage (when they might have unique functions) you certainly have to consider the anatomical location. But what if the main function for new neurons is not realized until they are fully mature? Well, if you plan to ablate or silence new neurons and examine behavioural effects, then you might want to wait longer if you’re planning on investigating emotion/stress-related behaviours that might rely more heavily on the temporal hippocampus. They also suggest that new neurons in the temporal dentate gyrus might have an extended unique role since they remain immature for longer. The peculiar survival difference between the infrapyramidal and suprapyramidal blades doesn’t do much to clarify the functions of these two regions, but it adds to the ever-growing list of differences that suggests they are truly distinct.
Often findings across labs do not match up as well as one would like so I am rather happy that Piatti et al. have found similar maturation differences in another species (mice) and using different methods (electrophysiological recordings from virally-labelled new neurons). So this is probably for real!
Lastly, a reviewer pointed out something very helpful, which is that it can be very difficult to discern the two blades of the dentate gyrus in caudal coronal sections (like this or those slightly more caudal where the dentate gyrus is essentially a blob). I spent a fair bit of time looking perplexed as I played with 3D paper models of the dentate gyrus but felt pretty cool doing so because similar strategies have been used by some of the foremost neuroanatomists of our time – see here. A 3D computer model that incorporated the blades of the dentate gyrus would have been very convenient (talking to you, Allen). In any case, we decided to remove our caudal blade analyses from the paper and instead only focussed on the septal infrapyramidal vs. suprapyramidal differences.
Reference: Snyder JS, Ferrante SF, Cameron HA (2012) Late Maturation of Adult-Born Neurons in the Temporal Dentate Gyrus. PLoS ONE 7(11): e48757. PMID: 23144957
¹in rodents
²in humans
Am very interested in your work on neurogenesis though am just getting a hang of it myself..I am currently researching for my seminar on new frontiers in neurogenesis at my university, the university of Nigeria. Would love it if u could assit me…it is a very wide and interesting field.
hi job well done. Pls am wrking on my MSc. Project wrk. I want to check d mode of cell death of tropane alkaloid induced neurodegeneration in rats. I need professional advice. Thanks. University of Ilorin