Today we have published a new review that was inspired by the controversy over whether adult neurogenesis occurs in humans: JS Snyder (2019) Trends in Neurosciences, DOI:https://doi.org/10.1016/j.tins.2018.12.001
ABSTRACT: Conflicting reports about whether adult hippocampal neurogenesis occurs in humans raise questions about its significance for human health and the relevance of animal models. Drawing upon published data, I review species’ neurogenesis rates across the lifespan and propose that accelerated neurodevelopmental timing is consistent with lower rates of neurogenesis in adult primates and humans. Nonetheless, protracted neurogenesis may produce populations of neurons that retain plastic properties for long intervals, and have distinct functions depending on when in the lifespan they were born. With some conceptual recalibration we may therefore be able to reconcile seemingly disparate findings and continue to ask how adult neurogenesis, as studied in animals, is relevant for human health.
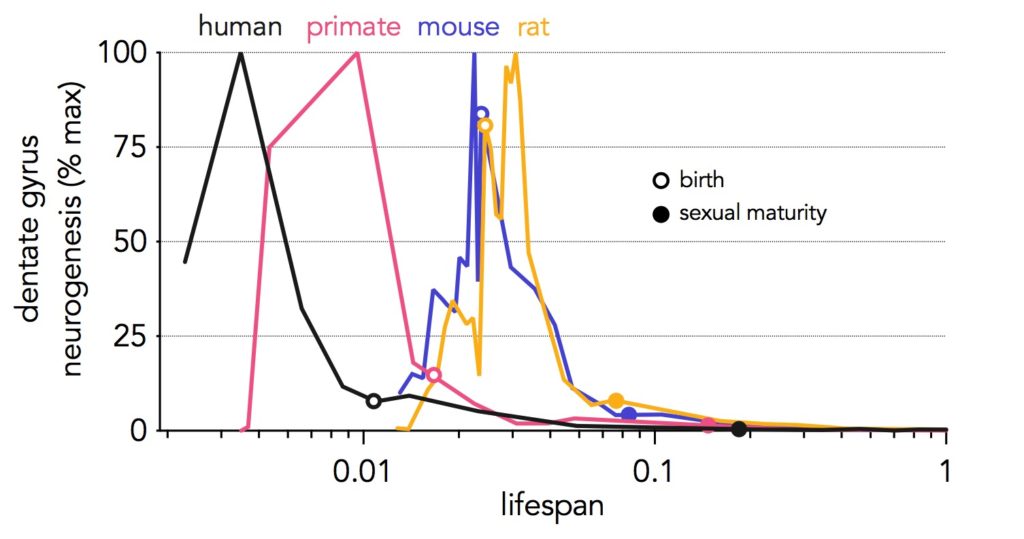
A number of reviews that have come out since the contradictory reports from Sorrells et al (human hippocampal neurogenesis ends in childhood; see also this post) and Boldrini et al (human hippocampal neurogenesis continues in adulthood). They have mainly focussed on methodological differences that could explain the discrepancy. This is an important conversation but I do not have the answer. So, here I instead chose to take a semi-quantitative look at the broader literature and see how neurogenesis across the lifespan aligns between animals and humans. My feeling was that, while there may be some unresolved issues in the field, there is likely some truth to each of the many studies that have come out over the past several years. Actually, I went as far back as the 1960s and 1970s to include some of the rare studies that have addressed key aspects of hippocampal development.
Here are the take home messages:
- Hippocampal (specifically dentate gyrus) neurogenesis occurs largely prenatally in humans and non-human primates whereas in rodents it peaks around the time of birth. The shift towards earlier stages of the lifespan suggests that indeed stem cells could “peter out” (to use precise scientific jargon) earlier in humans, resulting in less neurogenesis later in life.
- Nonetheless, for much of later adult life in ALL species, neurogenesis is very low, and so humans and animals are likely not that different.
- Why have these details been relatively underappreciated? It turns out that we, in the field, tend to study neurogenesis in young animals (when neurogenesis remains high) but older humans (when neurogenesis has declined).
- From this perspective, we could “recalibrate” the functional relevance of neurogenesis. Two scenarios immediately come to mind: 1) low rates of neurogenesis in adult humans, over a decade (for example) could still produce a meaningful difference (perhaps 15% of total neurons). 2) Higher rates earlier in life might mean that neurogenesis could play a more important role in childhood and adolescent development. Importantly, animal work suggests “adult” neurogenesis is involved in memory and emotional behaviors. Many relevant mental health disorders arise in young people, and so we might want to consider how neurogenesis might be involved in precipitating these disorders.
- Might neurogenesis, as studied in adult animals, still play an important role later in adult life in humans? Here, I think the answer is yes. But we need to do more experiments. One issue to date is the fact that we tend to study young neurons in young animals. The conventional wisdom is that newborn neurons become just like the older ones once they reach a certain age (~2 months old in rodents). However, recent findings indicate that some plastic and unique features of new neurons may persist much longer than that. Particularly in longer-lived animals (possibly including humans). So, even neurons born in childhood could continue to develop for many years, or even decades. But we need to study these older “newborn” neurons and not fixate only on the young ones.
- A second exciting possibility in that neurons born at different stages of life may remain functionally distinct, even once they are fully mature. This is the case in many other developing brain regions, so why not the dentate gyrus, where development is particularly drawn out? The paper discusses several cases of this and you can read about one of them here, where we found that, in contrast to old adult-born neurons, some old developmentally-born neurons die off in young adulthood. So, not all neurons are created equal. They may have different functions and they may also be differentially involved in susceptibility to diseases and disorders.
In sum, the field has gone through many controversies since the 1960s when adult neurogenesis was first discovered. A worst case scenario is one where we blindly accept that it simply occurs or does not occur, and is therefore relevant or irrelevant. Because then we stop asking new questions. Likely the truth lies somewhere in the middle, and by considering even the negative reports (disconcerting as they may be), we can be be stimulated to ask these new questions.
Is it a measurement problem?
https://www.nature.com/articles/s41591-019-0375-9
“Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease”
The control group was 13 neurologically healthy deceased people aged 43 to 87. The AD group was 45 deceased people, distributed among the six Braak stages of the pathology, aged 52 to 97.